Modeling of the electrical activity of the Helisoma trivolvis B5 neurons
Vladimir Kotov (Neuroinformatics Dpt. , NIISI RAS, RF), Liana Artinian (Biology Dpt, GSU, Atlanta, GA, USA), Vincent Rehder (Biology Dpt, GSU, Atlanta, GA, USA), Witali Dunin-Barkowski (Neuroinformatics Dpt. , NIISI RAS, RF)
The ability of neurons to produce spontaneous spiking depends on several small depolarizing currents activated at subthreshold voltages, such as the steady-state persistent sodium current, INaP, and the hyperpolarization-activated cyclic nucleotide-gated current, Ih. B5 neurons from the buccal ganglion of the pond snail Helisoma trivolvis, grown physically isolated in single cell culture, fire spontaneous tonic action potentials (APs) [1]. Using whole-cell recordings in current-clamp and voltage-clamp modes, there is preliminary evidence that B5 neurons have a TTX-insensitive INaP that is activated between -60 mV and -40 mV, low- and high voltage-activated Ca2+ currents, Ca2+-dependent K currents mediated via BK and SK channels, and a strong hyperpolarization-activated cation channel mediating Ih [1 and unpublished results]. In addition, B5 neurons are capable of generating Ca2+-dependent evoked APs in the absence of sodium ions [1].
Here we report initial results of modeling experiments, in which we used spike trains obtained from current clamp recordings as a template for a computerized modeling approach to characterize the contribution of individual ion conductances to spontaneous and evoked spike generation in B5 neurons.
For simulations, we used Matlab ODE code following the comprehensive description of [2]. Our model included the following ionic components: (1) persistent Na current( INaP); (2) fast sodium current INa(fast); (3) Delayed rectifying K+ current; (4) N-type Ca2+- current; (5) Small conductance calcium-activated K current (SK), and (6) hyperpolarization-activated current (Ih). The channel parameters in the model were hand-tuned to visually match the experimentally observed recordings of the membrane potential. We also modeled the influence of APs on the intracellular Ca concentration using a leaky integrator.
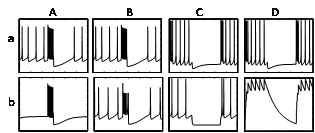
Modeling analysis confirmed the critical role of INaP in the generation of the spontaneous tonic firing activity. In the absence of INaP in the model (Ab), there was no spontaneous spiking when compared to the control (Aa), but APs could still be elicited through simulated current injection. When fast Na+ channels were removed from the model, B5 neurons continued to generate spontaneous and evoked spikes (Bb vs. Ba), supporting the notion that the APs were Ca mediated. Ih is characterized by its distinct sag-type response to hyperpolarizing current injection (Ca), and this current is absent when removed from the model (Cb). While Ih has been found to drive pacemaking activity in some mammalian neurons, elimination of Ih in B5 neurons did not block, but merely slowed spontaneous spiking in the model. Rebound spiking, occurring immediately after being released from a hyperpolarizing current injection is usually considered to depend on Ih. Rebound spiking in the model could not be fully explained by Ih, because some rebound spikes were still present without Ih in the model (Ca vs. Cb). Our model suggested another explanation for the hyperpolarization-induced increase in neuronal excitability. Modeling of the intracellular Ca concentration showed a marked reduction in the intracellular Ca2+ concentration (Db) induced by the hyperpolarizing current injection (Da). The decrease in intracellular Ca would be expected to cause the closing of Ca-activated K channels, resulting in an increase in excitability and causing rebound spiking. This prediction provides a testable hypothesis for the future electrophysiology experiments.
The work was supported by the RAS Presidium Program “Basic Sciences for Medicine” grant to W.D.B., and by NSF grant 0843173 to V.R. V.K. and W.D.B. were also partly supported by Federal Target Program “Scientific and scientific-pedagogical personnel of innovative Russia in 2009-2013” (contract no. P812).
References
[1] Artinian L., Tornieri K, Zhong L, Baro D, Rehder V. Nitric oxide acts as a volume transmitter to modulate electrical properties of spontaneously firing neurons via apamin-sensitive potassium channels. - J Neurosci. 2010, 30: 1699-1711.
[2] Purvis LK, Butera RJ Ionic current model of a hypoglossal motoneuron. J Neurophysiol. 2005, 93: 723-733.

 Latest news for Neuroinformatics 2011
Latest news for Neuroinformatics 2011 Follow INCF on Twitter
Follow INCF on Twitter