Network Analysis of Pain Processing: Pain is more than Sensation
Silke Kreitz (I. f. Pharmacology, FAU-Erlangen/Nuremberg, Germany), Marina Sergejeva (I. f. Pharmacology, FAU-Erlangen/Nuremberg, Germany), Andreas Hess (I. f. Pharmacology, FAU-Erlangen/Nuremberg, Germany)
Introduction: Modern neuroimaging techniques, such as functional MRI, made it possible to investigate the neural basis of pain perception non-invasively and at a high spatial resolution. Many studies have shown that nociceptive stimuli commonly elicit activity within a very wide array of subcortical and cortical brain structures [1,2]. These structures and their interactions are usually referred to as the “Pain matrix” and are considered to be specific for the processing of nociceptive stimuli [3,4]. Since recently this widely accepted theory is questioned: the so called “Pain matrix” is not specific for nociceptive stimulation but instead is involved in processing of salient input of different sensory modalities [5]. This theory relies mainly on the description of functions of neuronal units. Consequently, the investigation of the interregional connectivity comes into focus, providing additional information that might help to understand processing of sensory stimuli [6]. Therefore, we investigated the interregional connectivity specifically of pain related brain structures of mice for mild (sensory) and strong (nociceptive) heat stimuli.
Material and Methods: Experiments were performed on 10 wild-type male mice (10-14 weeks old, 1.2% isoflurane anesthesia at 37°C body temperature). Image data acquisition: fMRI experiments were performed with a 4.7 T/40 cm horizontal bore actively shielded magnet BioSpec (BRUKER, Germany). Gradient system (200 mT/m) and whole-body birdcage resonator enabled homogenous excitation. An actively RF-decoupled quadrature head coil was used as a receiver coil. Functional BOLD MRI scans were performed using a T2*-weighted single-shot gradient echo EPI sequence (22 axial slices, 64 x 64 matrix, TR= 2000 ms, k-space averaging of 2, TEef= 24.4 ms, field of view 15 x 15 mm, in-plane spatial resolution 234 x 234 μm, slice thickness 500 μm). Experimental protocol: A set of single thermal stimuli (45°,50°,55° and 60° ± 1°C, plateau for 5 s, ramp 15 s) was applied by a Peltier element on the right hindpaw three times in 3 min 25 sec intervals. Data processing: After motion correction, general linear modeling analysis with separate predictors for each stimulus, and false-discovery rate thresholding (q<0.05), different groups of activated voxels were labeled as belonging to 150 pain related brain structures based on the Paxinos mouse brain atlas [7]. Connectivity analysis: The average time courses of the activated voxels of each brain structure were corrected for global signal fluctuations by linear regression. Residual time courses were crosscorrelated individually for each stimulus and animal. The resulting crosscorrelation matrices (150x150) were averaged over all animals for each stimulus. Based on these mean crosscorrelation matrices NWB [8] was used to create stimulus specific networks with correlation coefficients as weights and brain structures as nodes. Networks communities were identified using the Blondel community detection [9].
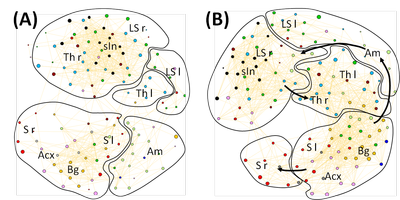
Results: The general pattern of the interregional connectivity network changes from sensory to nociceptive stimulation (Fig 1). Between the two highest temperatures no difference in the general network pattern could be observed (data not shown). During sensory stimulation the thalamus is separated into two different communities, representing the right and the left hemisphere. The amygdala complex is consolidated in an own community. The structures of the sensory cortex of both hemispheres belong to the same community. Nociception causes a unification of the thalamic structures of both hemispheres. The amygdala loses its compactness and communicates now with different brain regions including sensory input, hippocampus and basalganglia. The sensory cortex of the right hemisphere is separated from the sensory structures of the left hemisphere and forms an own community.
Disussion: The descriptive parameters such as size of activated area and activity show a linear increase over the stimulation temperatures [10] and thus are not sufficient to separate between sensory and nociceptive stimuli. The connectivity analysis performed in this study revealed a qualitative difference in the processing of nociceptive compared to sensory stimuli. This suggests that the processing of nociception may be indeed different from the processing of other salient stimuli.
References: [1] Brooks J & Tracey I (2005) J.Anat. 207:19-33 [2] Hess A et al. (2007) Eur J Pain 11:109-119 [3] Ploghaus A et al. (1999) Science 284:1979-1981 [4] Tracey I (2005) Curr Opn Neurobiol 15:478-487 [5] Ianetti GD & Mouraux A (2010) Exp Brain Res 205:1-12 [6] Schwarz et al. (2008) Magn Res Im 26: 914-920 [7] Paxinos G & Franklin KBJ (2001) The Mouse Brain in Stereotactic coordinates Burlington MA: Elsevier Academic Press [8] NWB team (2006) Network Workbench Tool. Indiana Univ and Northeastern Univ. [9] Blondel VD (2008), J Stat Mech-Theo 10:10008-10020 [10] Heindl-Erdmann C. et al. (2010) NeuroReport 21(1):29-33
Acknowledgement: BMBF (BCCN 01GQ0731, 0314102), and by the Doerenkamp Foundation for Innovations in Animal and Consumer Protection, ELAN grant from FAU.

 Latest news for Neuroinformatics 2011
Latest news for Neuroinformatics 2011 Follow INCF on Twitter
Follow INCF on Twitter